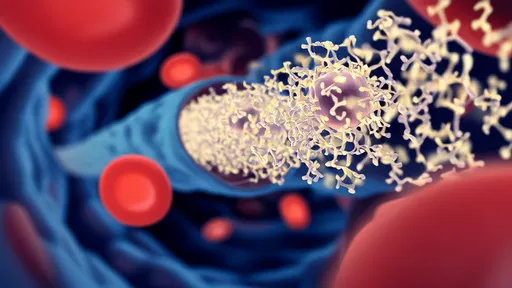
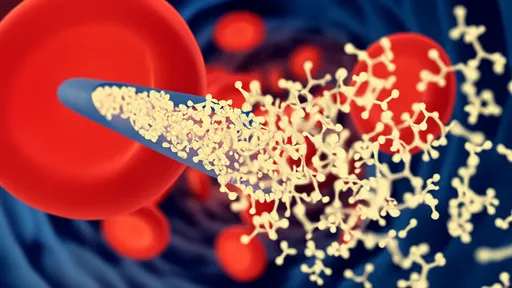
For decades, the blood-brain barrier (BBB) has stood as a formidable gatekeeper, shielding the brain from harmful substances while frustrating efforts to deliver life-saving therapeutics. This selective membrane, while essential for protecting neural tissue, has rendered nearly 98% of potential neuroactive drugs ineffective due to their inability to penetrate its defenses. Now, a groundbreaking approach leveraging artificial intelligence has yielded a potential master key—a class of engineered peptides capable of shuttling payloads across this biological fortress.
The newly designed peptides, dubbed "Brain Key" by researchers, emerged from an ambitious collaboration between computational biologists and neuroscientists. Unlike traditional drug development methods that rely on trial-and-error, the team employed deep learning algorithms trained on thousands of known peptide sequences and their BBB permeability data. The AI system identified subtle patterns in molecular structures that correlate with successful barrier penetration—patterns too complex for human researchers to discern.
What makes these peptides remarkable is their dual functionality. Not only do they demonstrate unprecedented BBB crossing efficiency in animal models, but their modular design allows conjugation with various therapeutic molecules. Early experiments show the shuttle peptides successfully transporting antibodies, enzymes, and even gene-editing tools into brain tissue while maintaining the cargo's biological activity. This versatility opens doors for treating conditions ranging from neurodegenerative diseases to brain tumors that have long resisted conventional treatment approaches.
The research team drew inspiration from nature's own BBB penetrators. Certain viruses and toxins evolved peptides that trick the barrier's transport systems, while natural human proteins like transferrin gain entry through receptor-mediated pathways. The AI-synthesized peptides appear to combine these strategies, exhibiting both passive membrane permeability and the ability to hijack active transport mechanisms. This biomimetic approach likely explains their superior performance compared to previous synthetic attempts.
Safety profiles from preliminary studies appear promising. Unlike some earlier BBB-penetrating technologies that caused inflammation or barrier disruption, the new peptides show excellent biocompatibility in primate models. Their relatively small size (12-20 amino acids) minimizes immune recognition while allowing for cost-effective synthesis. Perhaps most importantly, the peptides demonstrate precise brain targeting, with minimal accumulation in peripheral organs—a critical factor for reducing systemic side effects.
Clinical applications are already taking shape. Pharmaceutical companies have licensed the technology to develop targeted therapies for Alzheimer's disease, with plans to conjugate the shuttle peptides with beta-amyloid clearing antibodies. Oncology researchers are exploring its potential for delivering chemotherapy agents directly to brain metastases, potentially avoiding the debilitating cognitive side effects of whole-body treatment. The platform's modular nature means that as new neurotherapeutics emerge, the shuttle system could potentially adapt to deliver them.
While enthusiasm runs high, the researchers caution that significant challenges remain. Scaling up production while maintaining peptide stability presents engineering hurdles. The team is also working to further optimize the system's pharmacokinetics, as some early variants showed rapid clearance from circulation. Long-term studies will be needed to fully assess potential immunological responses with chronic use.
This AI-driven approach represents more than just another drug delivery technology—it fundamentally changes how we approach neurological drug development. By solving the BBB challenge, researchers can now reconsider thousands of previously discarded neuroactive compounds that failed solely due to delivery issues. The same computational platform used to design the Brain Key peptides is already being adapted to create transporters for other biological barriers, potentially revolutionizing treatment for ocular, placental, and even intracellular targets.
As the first clinical trials prepare to launch next year, the scientific community watches with cautious optimism. If successful, this technology could mark a turning point in our centuries-long struggle to effectively treat brain disorders. The implications extend beyond medicine—neuroscience research could benefit from more precise delivery of imaging agents and neural probes, accelerating our understanding of the brain itself. In unlocking the blood-brain barrier, we may ultimately unlock mysteries of the mind that have eluded us for generations.
By /Aug 18, 2025
By /Aug 18, 2025
By /Aug 18, 2025

By /Aug 18, 2025

By /Aug 18, 2025

By /Aug 18, 2025

By /Aug 18, 2025

By /Aug 18, 2025

By /Aug 18, 2025
By /Aug 18, 2025

By /Aug 18, 2025
By /Aug 18, 2025
By /Aug 18, 2025
By /Aug 18, 2025
By /Aug 18, 2025
By /Aug 18, 2025
By /Aug 18, 2025

By /Aug 18, 2025
By /Aug 18, 2025
By /Aug 18, 2025