In a groundbreaking development that could redefine aging and age-related disease treatment, scientists have unveiled a novel "smart bullet" technology designed to precisely eliminate senescent cells—the so-called "zombie cells" that accumulate with age and contribute to tissue dysfunction. This innovative approach, termed lysosome-targeted activating nanocapsules (LTAN), represents a quantum leap in targeted senolytic therapies, combining the precision of biological targeting with the controlled release capabilities of advanced nanomaterials.
The research, published in Nature Biomedical Engineering, details how these nanocapsules can distinguish between healthy and senescent cells with remarkable accuracy. Unlike previous senolytic drugs that often caused collateral damage to healthy tissues, the LTAN system specifically recognizes the unique lysosomal characteristics of zombie cells. "It's like having a microscopic detective that identifies the molecular fingerprints of senescence before delivering its payload," explains Dr. Elena Vostrikova, lead author of the study from the Swiss Federal Institute of Technology.
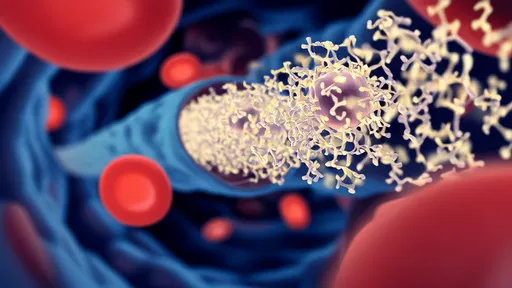
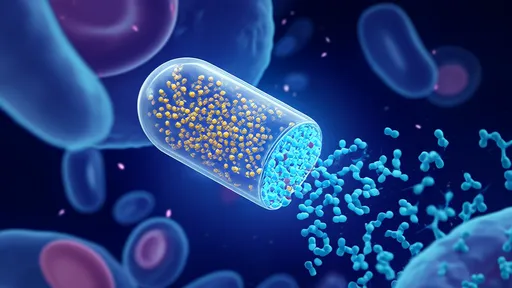
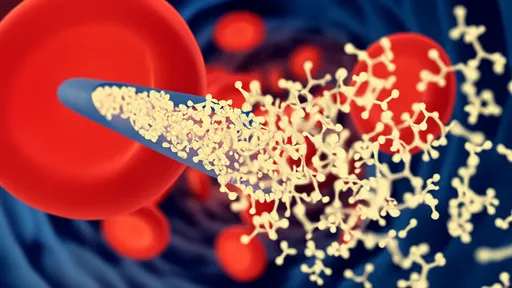
What makes this technology particularly revolutionary is its dual-action mechanism. The nanocapsules' outer shell contains antibodies that bind exclusively to laminin-1—a protein overexpressed on senescent cell membranes. Once internalized, the acidic environment of the lysosome triggers the nanocapsule to release its encapsulated drug cocktail. This cocktail includes a lysosomotropic agent that destabilizes the lysosomal membrane and a senolytic compound that induces selective cell death. The process creates a domino effect: lysosomal rupture initiates apoptosis specifically in the target cells while leaving neighboring healthy cells unharmed.
Preclinical trials have yielded exceptionally promising results. In aged mouse models, a single dose of LTANs reduced senescent cell burden by 78% in adipose tissue and 64% in skeletal muscle within 48 hours—far surpassing the efficacy of existing senolytics like dasatinib and quercetin combinations. Perhaps more impressively, treated animals showed significant improvements in physical function, with grip strength increasing by 41% and treadmill endurance doubling compared to control groups. "The animals didn't just live longer—they lived better, with restored vitality that mirrored much younger subjects," notes Dr. Vostrikova.
The implications for human health are profound. Senescent cells have been implicated in nearly every age-related condition from osteoarthritis to atherosclerosis, and their removal has shown potential to not just treat but potentially reverse certain aspects of aging. Current estimates suggest LTAN therapy could enter human clinical trials within three years, initially targeting severe fibrotic lung diseases where senescent cells play a well-documented role. Pharmaceutical giants have already begun licensing negotiations, recognizing this as potentially the first true anti-aging medication rather than merely symptomatic treatment.
However, challenges remain before widespread clinical application. Researchers must confirm long-term safety, particularly regarding potential off-target effects during chronic administration. There are also open questions about optimal dosing frequency—whether periodic "clean-outs" or continuous low-level suppression proves more effective. The team is currently developing second-generation LTANs with additional targeting moieties to address rare senescent cell subtypes that may evade the current formulation.
Beyond aging, this technology platform could be adapted for other precision medicine applications. The same lysosomal targeting principle might deliver drugs selectively to cancer cells or infected cells. Some researchers speculate about engineering LTANs to target specific senescent cell populations—for instance, just those contributing to Alzheimer's pathology while sparing cells involved in wound healing. "We're not just looking at a single drug, but at an entirely new paradigm for cellular-scale precision medicine," concludes Dr. Vostrikova.
As the global population ages, the economic and humanitarian potential of effectively addressing senescence becomes increasingly apparent. If human trials replicate the animal results, LTAN technology could transform geriatric medicine, potentially adding not just years to life but life to years. The researchers caution that immortality remains science fiction, but significant healthspan extension now appears within scientific reach—all thanks to these microscopic "smart bullets" that seek out and destroy our cellular zombies.
By /Aug 18, 2025
By /Aug 18, 2025
By /Aug 18, 2025

By /Aug 18, 2025

By /Aug 18, 2025

By /Aug 18, 2025

By /Aug 18, 2025

By /Aug 18, 2025

By /Aug 18, 2025
By /Aug 18, 2025

By /Aug 18, 2025
By /Aug 18, 2025
By /Aug 18, 2025
By /Aug 18, 2025
By /Aug 18, 2025
By /Aug 18, 2025
By /Aug 18, 2025

By /Aug 18, 2025
By /Aug 18, 2025
By /Aug 18, 2025