In a groundbreaking development that could revolutionize spinal cord injury treatment, scientists have successfully demonstrated the use of magnetically controlled nanorobots to reconstruct neural pathways in damaged spinal cords. This innovative approach, dubbed "magnetic neural weaving," represents a significant leap forward in regenerative medicine and offers new hope for patients with paralysis.
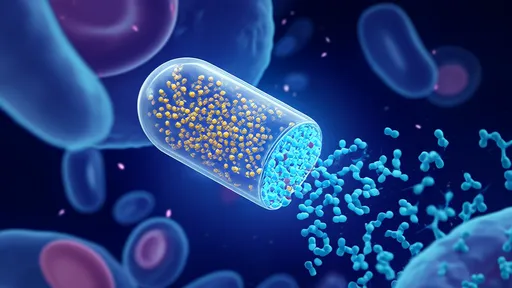
The research team from the Swiss Federal Institute of Technology (EPFL) has developed microscopic robots capable of navigating through the delicate environment of the central nervous system. These nanoscale devices, no larger than a red blood cell, are guided by precisely controlled magnetic fields to perform their delicate surgical work at the cellular level. What makes this technology particularly remarkable is its ability to operate without physical tethers or invasive procedures, minimizing trauma to surrounding healthy tissue.
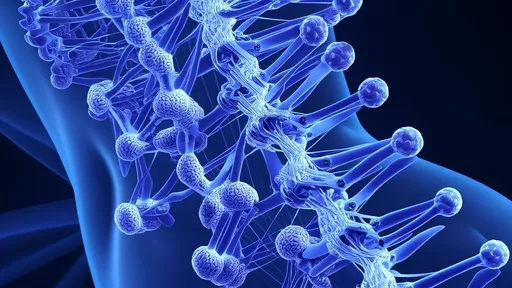
Spinal cord injuries have long presented an insurmountable challenge for medical science. When the spinal cord is damaged, the neural pathways that carry signals between the brain and the rest of the body are severed, often resulting in permanent loss of function below the injury site. Traditional approaches have focused on preventing further damage and managing symptoms, but the EPFL team's work represents one of the first practical attempts to actually rebuild these critical neural connections.
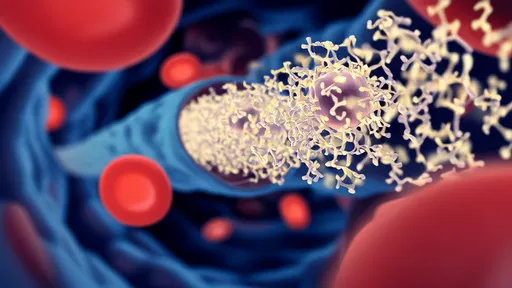
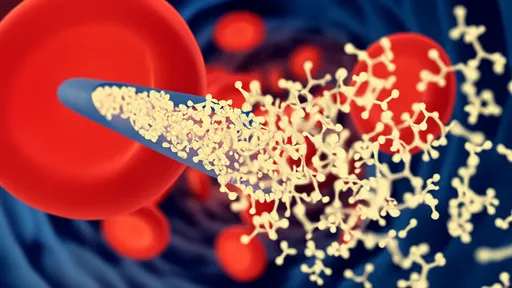
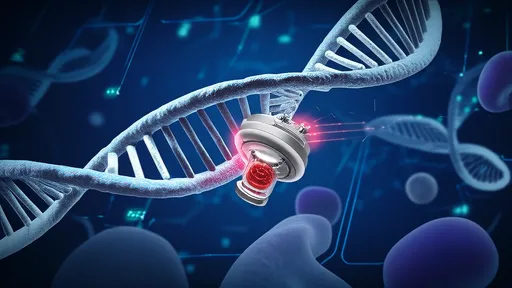
The magnetic nanorobots function as microscopic construction workers in the nervous system. Coated with special proteins that promote nerve growth, these devices can physically guide the regeneration of axons - the long, slender projections of nerve cells that transmit electrical impulses. By creating a sort of "magnetic scaffolding," the robots help bridge the gap created by spinal injuries, allowing nerve fibers to reconnect across damaged areas.
Professor Marco Dorigo, who led the research team, explains: "Imagine trying to repair a complex telephone network where thousands of wires have been cut. Our nanorobots act like microscopic technicians, carefully guiding each wire back to its proper connection point. The magnetic control allows us to position them with incredible precision and make adjustments in real-time as the repair work progresses."
The technology builds upon recent advances in several fields simultaneously. From materials science comes the development of biocompatible, magnetic nanoparticles that can safely operate within living tissue. Neuroscience contributions include improved understanding of nerve regeneration mechanisms. The control systems borrow from cutting-edge robotics and artificial intelligence research, allowing for precise navigation through the body's complex terrain.
Early animal trials have shown particularly promising results. In tests with rats that had complete spinal cord transections - the most severe type of spinal injury - subjects treated with the nanorobot system regained significant motor function. While not yet achieving full recovery, the improvement was substantially better than any existing treatment option. Perhaps most encouragingly, the regenerated neural connections appeared stable over time, suggesting the repairs could be permanent.
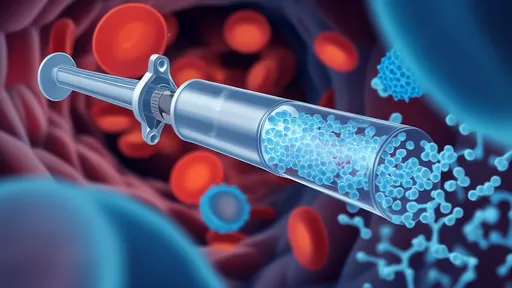
The procedure begins with an injection of millions of these microscopic robots into the cerebrospinal fluid near the injury site. Using external magnetic fields controlled by sophisticated computer systems, surgeons then guide the robots to the precise locations where they're needed. Some robots serve as anchors, holding tissue in optimal positions for repair, while others actively guide growing nerve fibers across the injury gap.
One of the most significant challenges the team overcame was developing a control system precise enough to manipulate objects at this scale within the body's constantly shifting environment. The solution came in the form of real-time MRI guidance combined with advanced algorithms that can compensate for natural movements like breathing and blood flow. This allows the magnetic fields to be adjusted hundreds of times per second, keeping the nanorobots on course despite the body's internal dynamics.
Safety considerations have been paramount throughout the development process. The nanorobots are designed to be completely biodegradable, breaking down into harmless components once their work is complete. Extensive testing has shown no evidence of immune rejection or other adverse effects in animal models. The magnetic fields used for control are well within established safety limits and cause no detectable heating or tissue damage.
Looking ahead, the research team is working to refine the technology for human trials. Current efforts focus on scaling up the system for the larger human spinal cord and developing more sophisticated control interfaces for surgeons. While significant work remains, the researchers estimate that clinical applications could be possible within the next 5-7 years if progress continues at its current pace.
The potential implications extend beyond spinal cord injuries. The same basic technology might eventually be adapted to repair peripheral nerve damage, treat certain neurodegenerative conditions, or even help interface prosthetic limbs directly with the nervous system. Some researchers speculate that similar approaches could one day be used to create direct connections between neural tissue and advanced computer interfaces.
As with any emerging medical technology, numerous questions remain to be answered. Long-term effects, optimal treatment protocols, and potential limitations will need to be thoroughly investigated through rigorous clinical testing. However, the scientific community has greeted these initial results with cautious optimism, recognizing their potential to transform treatment paradigms for conditions once considered permanently debilitating.
For the millions worldwide living with spinal cord injuries, this research offers something precious: renewed hope. While not yet a cure, it represents one of the most promising avenues yet discovered for restoring function after devastating neurological damage. As research progresses, the vision of doctors using tiny magnetic robots to repair broken neural pathways moves steadily closer to reality.
By /Aug 18, 2025
By /Aug 18, 2025
By /Aug 18, 2025

By /Aug 18, 2025

By /Aug 18, 2025

By /Aug 18, 2025

By /Aug 18, 2025

By /Aug 18, 2025

By /Aug 18, 2025
By /Aug 18, 2025

By /Aug 18, 2025
By /Aug 18, 2025
By /Aug 18, 2025
By /Aug 18, 2025
By /Aug 18, 2025
By /Aug 18, 2025
By /Aug 18, 2025

By /Aug 18, 2025
By /Aug 18, 2025
By /Aug 18, 2025