In a groundbreaking fusion of biotechnology and photonics, researchers have unveiled a novel "DNA photodrone" system capable of delivering CRISPR gene-editing tools to precise brain targets using near-infrared light. This innovative approach promises to revolutionize the treatment of neurological disorders by overcoming the blood-brain barrier and achieving unprecedented spatial-temporal control over gene editing.
The system, developed by an international team from MIT and Seoul National University, exploits the unique properties of near-infrared (NIR) light to penetrate deep into biological tissues. Unlike conventional CRISPR delivery methods that rely on viral vectors or nanoparticles with limited targeting capabilities, this photonic approach enables remote activation of gene editing precisely where and when it's needed. The implications for treating conditions like Parkinson's, glioblastoma, and epilepsy are profound, as clinicians could theoretically correct genetic mutations or regulate neural activity without invasive procedures.
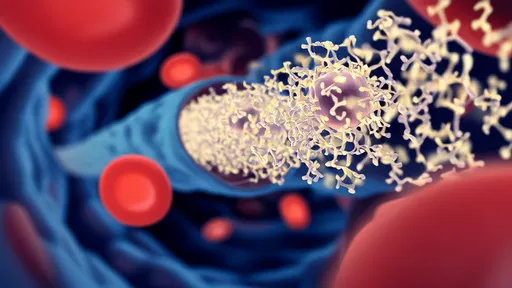
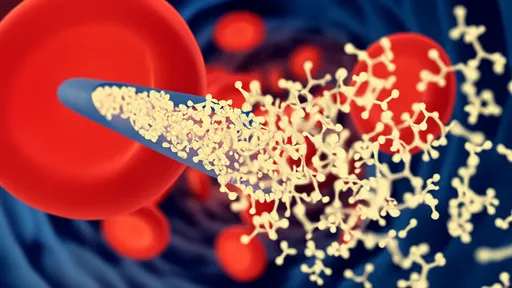
At the core of this technology lies a sophisticated DNA nanostructure that serves as both carrier and activator. These pyramid-shaped "photodrones" remain biologically inert until illuminated by specific NIR wavelengths (700-900 nm). When activated, they undergo a conformational change that exposes the CRISPR-Cas9 machinery, allowing it to edit target cells within a precisely defined volume. This light-triggered mechanism provides millimeter-scale spatial resolution - a critical advantage when working with delicate neural circuits where off-target effects could prove disastrous.
The researchers faced significant challenges in engineering the photodrones' light sensitivity. Through extensive molecular modeling, they identified optimal DNA origami configurations that respond predictably to NIR stimulation while maintaining stability in cerebrospinal fluid. Particular attention was paid to the photon absorption characteristics, ensuring sufficient energy transfer to trigger the structural transition without generating harmful heat. Animal trials demonstrated 83% target engagement accuracy in cortical tissue, with minimal dispersion beyond the illuminated zone.
Clinical applications appear particularly promising for treating focal epilepsy. Current anti-seizure medications affect the entire brain, often causing debilitating side effects. The photodrone system could allow doctors to correct hyperexcitable neural circuits at their source. Similarly, in glioblastoma cases, the technology might enable selective knockout of oncogenes in tumor margins while sparing healthy tissue - a capability impossible with current chemotherapy approaches.
Beyond therapeutic uses, the platform opens new avenues for neuroscience research. Precise genetic manipulation of specific neuronal populations could help unravel the molecular basis of memory formation, addiction pathways, and neurodegenerative processes. The team has already begun adapting the system for optogenetic applications, potentially creating a unified platform for both gene editing and neural activity modulation.
While regulatory approval remains years away, the technology has attracted significant interest from pharmaceutical companies and medical device manufacturers. The researchers estimate human trials could begin within 3-5 years, pending successful completion of large-animal safety studies. As the team refines the photodrone's targeting specificity and develops companion imaging systems for real-time guidance, what began as a proof-of-concept may soon transform how we treat the most challenging brain disorders.
Ethical considerations surrounding light-controllable gene editing are already being discussed in academic circles. Unlike traditional CRISPR that's active immediately upon delivery, this system's spatial and temporal control could mitigate some concerns about unintended genomic alterations. However, the potential for covert applications or military use has prompted calls for preemptive governance frameworks. The developers have proactively engaged with bioethics organizations to establish responsible research guidelines as the technology progresses.
From an engineering perspective, future iterations aim to incorporate multiple wavelength sensitivity, allowing sequential activation of different genetic programs. Early work shows promise for combinatorial therapies where one photodrone payload might first knock out a disease gene, then activate a therapeutic replacement - all controlled by distinct light pulses. Such capabilities could make the system invaluable for complex polygenic disorders that currently defy treatment.
The convergence of photonics and nanotechnology in this project highlights the increasing interdisciplinary nature of medical breakthroughs. As research continues, the team is exploring partnerships with optical device companies to develop compact, clinically viable light delivery systems. The ultimate vision involves a fully implantable device that could provide chronic condition management through periodic light-activated gene regulation - a futuristic approach that's suddenly looking increasingly attainable.
By /Aug 18, 2025
By /Aug 18, 2025
By /Aug 18, 2025

By /Aug 18, 2025

By /Aug 18, 2025

By /Aug 18, 2025

By /Aug 18, 2025

By /Aug 18, 2025

By /Aug 18, 2025
By /Aug 18, 2025

By /Aug 18, 2025
By /Aug 18, 2025
By /Aug 18, 2025
By /Aug 18, 2025
By /Aug 18, 2025
By /Aug 18, 2025
By /Aug 18, 2025

By /Aug 18, 2025
By /Aug 18, 2025
By /Aug 18, 2025